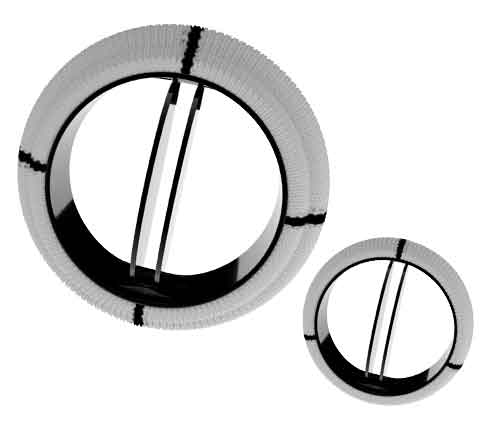
The MILTONIA Mechanical Heart valve is a Bi-Leaflet Heart Valve made up of highly durable Pyrolytic Carbon on a Graphite substrate. The Pyrolytic Carbon is Silicon (Si) alloyed and contains around 3 to 8 % of Silicon(Si) and around 12% Tungsten(W) for radiopacity.
The Polished Silicon alloyed varieties of Pyrolytic Carbon exhibit an excellent degree of thromboresistance while improving resistance to wear, offering excellent and durable results.
An open pivot design provides a Passive Washing mechanism of pivot which minimizes thrombolytic events. The Orifice ring consists entirely of Pyrolytic Carbon. In the full open position, the plane of each leaflet forms a nominal angle of 85° relative to the plane of the orifice ring.
The valve Sewing cuff is made up of Double Velour Polyester fabric and is mounted on the orifice using a Titanium(Ti) Stiffening ring and secured with two Titanium(Ti) Lock rings and a Lock wire. This method of Sewing cuff attachment to the orifice allows for rotation of the carbon orifice in situ during implantation. Three markers are located in the aortic Sewing ring and four markers are located in the mitral sewing ring to assist in the uniform placement of sutures around the valve annulus.
Overview: The Miltonia Coronary Stent System is an advanced solution for coronary revascularization, combining state-of-the-art technology with proven clinical outcomes to restore vascular health effectively. Key Features: Innovative Design: The stent's hybrid design ensures optimal scaffolding and flexibility. Drug-Coated Efficacy: Sirolimus drug-eluting system minimizes restenosis and promotes healing. Ease of Deployment: Enhanced balloon-expandable delivery system ensures precision and control. Material Quality: Manufactured with cobalt chromium for superior strength and visibility under imaging. Specifications: Available sizes: Stent diameters: 2.0 mm - 4.5 mm; Lengths: 8 mm - 38 mm. Drug Coating: Sirolimus, with a controlled-release polymer matrix. Delivery System: Compatible with standard 0.014" guidewires and low-profile catheter systems.
NOTE: Meril Life Sciences India Pvt. Limited (Trademarks) is having full rights and responsibilities about product design, specifications, performance, obligation and etc…
Dasherz is not responsible for product design, specifications, performance, obligation and etc…
Dasherz use its best efforts to promote the products and maximize the sale of the products in the Territory of states of Odisa by displaying products..